Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
:max_bytes(150000):strip_icc():format(jpeg)/SinuCleanse-Soft-Tip-Squeeze-Bottle-Nasal-Wash-System-022625-tout-37d89f355ae74d3dbe0dd085b011c361.jpg)
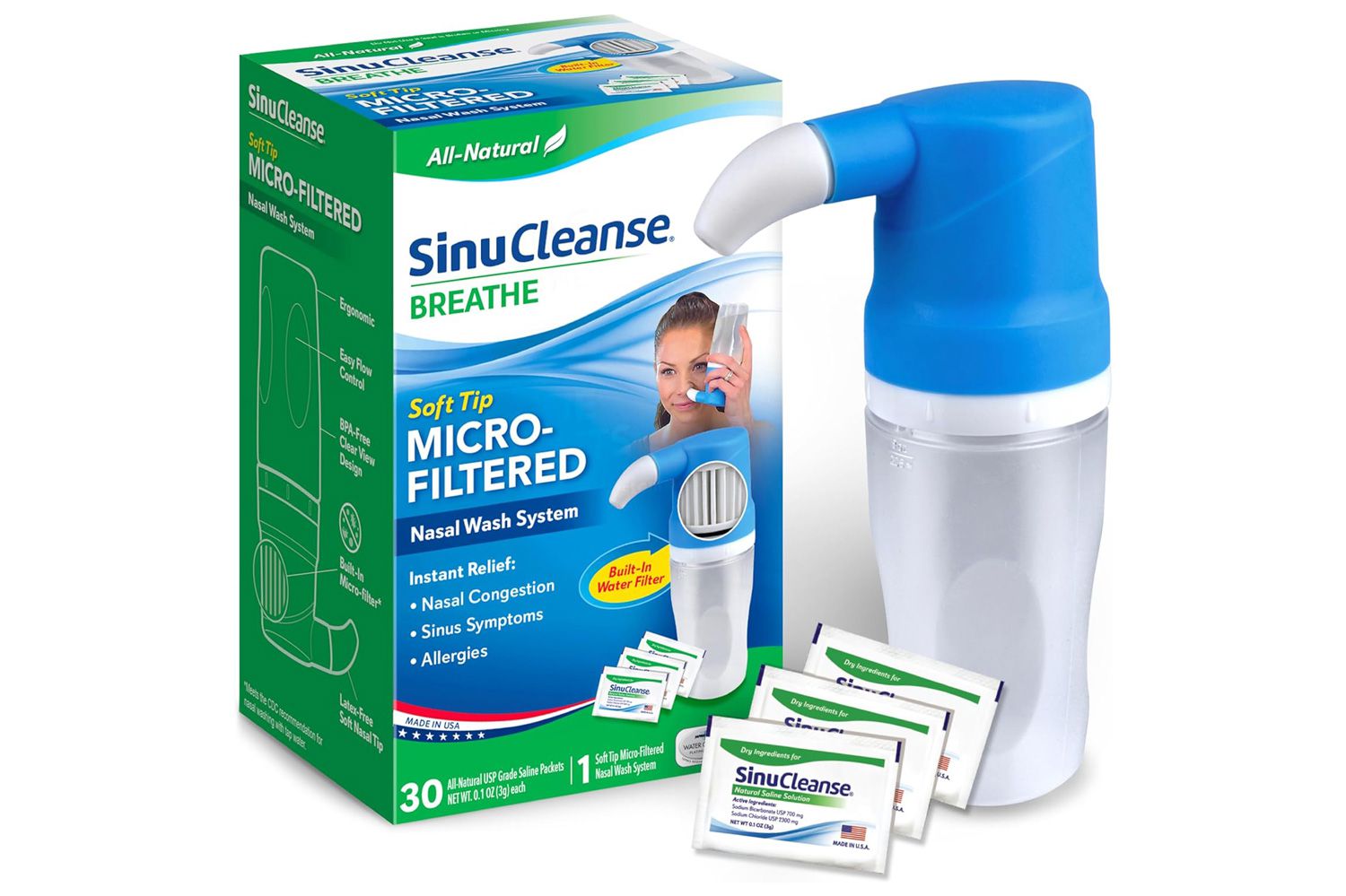
A cold and influenza nasal laundry product is recalled after it tested positively for a dangerous bacterium.
ASCENT Consumer Products Inc. announced a voluntary recall On the FDA website on Tuesday, February 24 of its Sinucleanse Soft Tip Squeeze Nasal Wash System after a test result confirmed that there was a “microbial pollution of the product with Staphyloccus Aureus.”
According to Centers for Disease Control (CDC), Staphylococcus aureus is a bacteria “that is usually found on the skin and in the nose of about 30% of individuals” that can cause skin infections, or if it enters the body, can lead to “severe or deadly” symptoms.
The recall affects Sinucleanse Soft Tip Squeeze Nasal Wash System with an expiration date of December 31, 2027. The product is described as “a nest washing in the nasal passages to temporarily alleviate symptoms associated with sinusine, cold, influenza or allergies.”
The product is packed in a cardboard box containing a “Squeeze bottle and 30 salt packs”, and it was distributed across the United States both in stores and online in January 2025, according to the company. The product’s party is “024122661A1.”
Ascent Consumer Products Inc. found that people “whose nasal mucosa can be compromised due to inflammation and mechanical damage” who use their product contaminated with the bacteria can have a blood infection.
Never miss a story – register for People’s free daily newsletters Keeping up to date on the best of what people have to offer, from celebrity news to compelling stories of human interest.
People can also get “secondary infections” including endocarditis, an “infection of the heart’s internal feed”, “bone and joint infections” or “bacterial sinusitis”, which according to the recall “can lead to eye tissue infections, vision problems, cranial nerve injuries or meningitis.”
“These infections are serious and potentially life -threatening,” the company said.
So far, “no side effects” have been reported to Aspecent Consumer Products Inc. but in relation to the recall, the company says.
Customers who bought the product have been asked to cancel it and immediately return it where it was purchased or discard it. The company noted that if customers “experience any problems related to the use of this product”, they should contact their doctor or healthcare provider.
Distributors were also contacted by the company and asked to “cease distribution” and “remove” the products from their warehouse.